NEW GENERATION BIOTECH AND HEALTH TECHNOLOGY
MULTI-DIRECTIONAL RESEARCH AND DEVELOPMENT ACTIVITIES
ABOUT
KHERION
Kherion is a new generation biotech and health technology company and have four main research and development areas including cellular and gene therapy, tissue engineering/biomaterial, functional foods and nutritional supplements and biomarker panels.
Kherion is following scientific developments, research and development activities aimed at developing cellular and gene therapy products for use in the treatment and prevention of diseases perform studies on developing stem cells, cellular products, tissue products, extracellular vesicles and immunotherapeutic products from different tissue sources.

- Active market access (3)
OUR SCIENCE
1. INITIATIVE
the impetus for development comes from collaborating physicians who lack a suitable solution for a particular medical indication.
2. POTENTIAL
benefits and risks for new product development are evaluated internally within the company
3. DEVELOPMENT
a development team works in the company's laboratories on the form and design of the new product, throughout the development process we are in close contact with specialists for the medical indication
4. TRANSLATION
the last stage of development is the transfer of the product into production practice, our products are manufactured according to GMP standards
2. POTENTIAL
benefits and risks for new product development are evaluated internally within the company
4. TRANSLATION
the last stage of development is the transfer of the product into production practice, our products are manufactured according to GMP standards
PIPELINE
Technology | Indication | Program | Phase 1 | Phase 2 | Phase 3 | Market entry |
TECHSYMPHONY® | Diabetic Foot Ulcers |
AMNIODERM+®
| EU-2023 |
Acute and chronic wounds |
AMx23
| EU-2025 | |
Gynecology |
AMNIOBARRIER®
| EU-2025 | |
Eye surgery |
AMx1102
| EU-2025 |
AMNIPUR® | Diabetic Foot Ulcers |
AMNIODERM®
| on market |
Acute and chronic wounds |
AM_Nx
| on market | |
Epidermolysis Bullosa |
AM_EBC
| EU-2023 | |
Traumatology |
UMBIFIX®
| EU-2024 |
PUROTECH® | Eye surgery |
AMNIOEYE®
| on market |
AmnioDisc® (AMt23)
| EU-2023 |
OUR PRODUCTS

AMNIODERM+®
- Solid patch or gel
- Chronic and acute injuries, burns
- Venous leg ulcers
- Arterial skin ulcers
- Pressure ulcers
- Neuropathic skin ulcers
- Lymphedema ulcers
AMNIODERM+®
Current product status: Product in clinical trial
Method of administration: topical application, administered by a healthcare professional
Form: sterile, in gel or solid patch, different volumes
Shelf life: 5 years, room temperature
MECHANISM OF ACTION
EXAMPLES OF USE
Wound Dressing is intended for the management of wounds including:
- partial and full-thickness
- wounds
- pressure ulcers
- venous ulcers
- diabetic ulcers
- chronic vascular ulcer
- surgical wounds
- trauma wound
WARNINGS AND PRECAUTIONS
- BH-Wound Dressing is not intended for use on third-degree burns.
- Do not resterilize
- Do not use if the package seal is broken.
- BH-Wound Dressing should not be applied until excessive exudate, bleeding, acute swelling and infection are controlled.
- Debridement or excision must be done thoroughly to remove any remaining necrotic tissue that may cause infection.
- Do not stretch, expand, spread or mesh the device.
OUR EXPERIENCE

UMBIFIX®
- Mesh, cryopreserved patch
- Achilles tendon rupture
UMBIFIX®
Current product status: Product under development
Method of administration: topical application, administered by a doctor
Form of product: Sterile deep-frozen product, various sizes
Shelf life: 2 years, room temperature

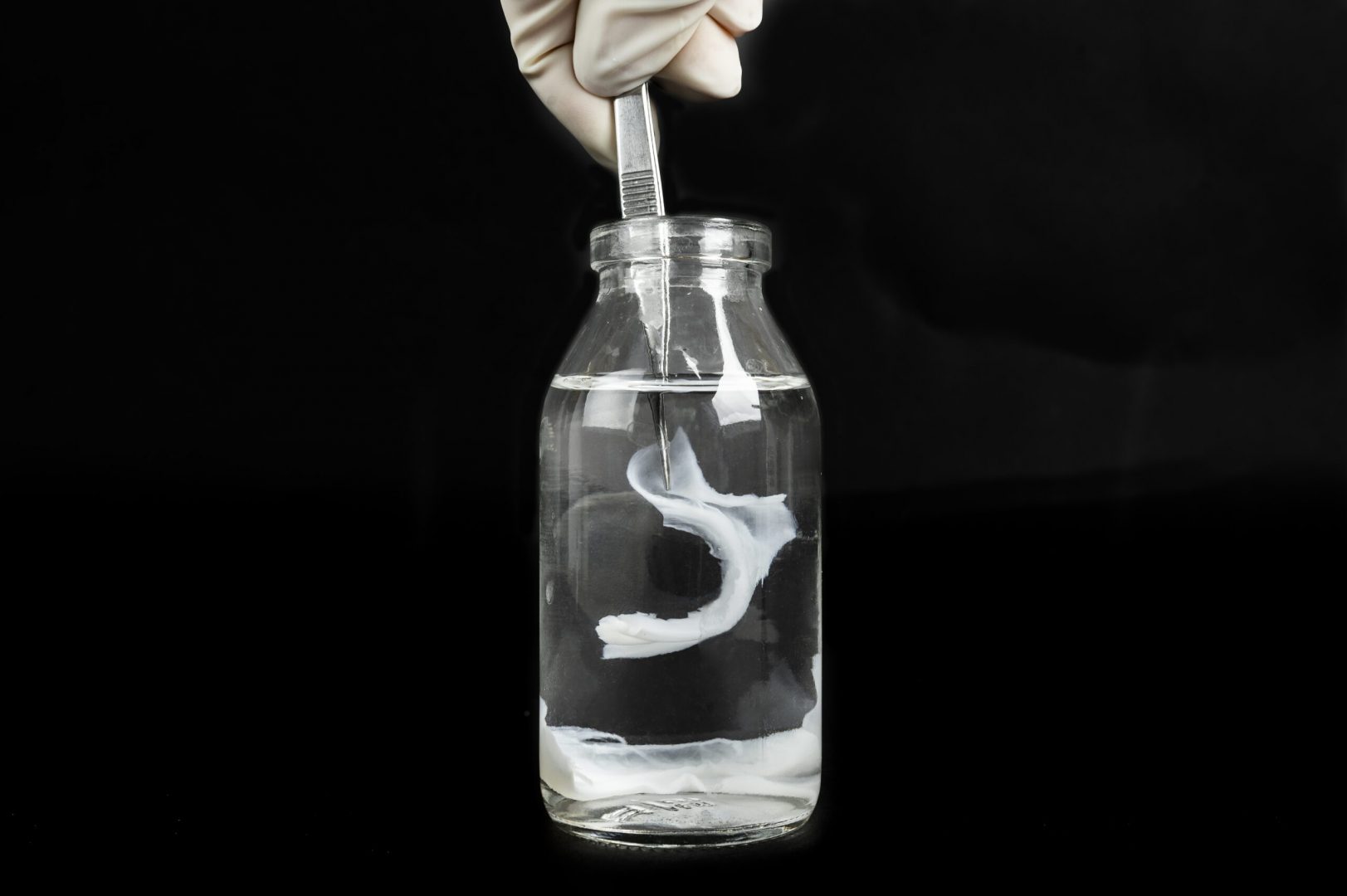

MECHANISM OF ACTION
The amniotic membrane from the umbilical cord is of entirely biological origin and contains a large number of bioactive molecules, including hyaluronic acid and MSC.
There is currently no product on the Czech or European market that uses this tissue to facilitate healing or increase the effectiveness of surgery and prevent repeat rupture of the Achilles tendon. Rupture of the Achilles tendon is treated by conservative or surgical method without further interventions. By contrast, the newly-developed product offers the patient and the health and social system the following benefits:
- Suitable for both classic and robotic surgery
- Adapts to the tendon, adheres perfectly to the wounded tissue
- Umbilical cord tissue can be stitched on to the affected area
- Reduced regeneration time
- 100% functionality of the damaged tendon
- Simple-design products that the operator will have at hand at the moment of need
- Preventing permanent consequences and ensuring a full life
OUR EXPERIENCE
Once development of the product has been completed, the patient will have a choice of two options – taking the classic road as before, or choosing above-standard application of the developed product and in doing so actively promoting the reduction of recovery time.
EXAMPLES OF USE
- Achilles tendon rupture
MARKET AUTHORISATION 2021
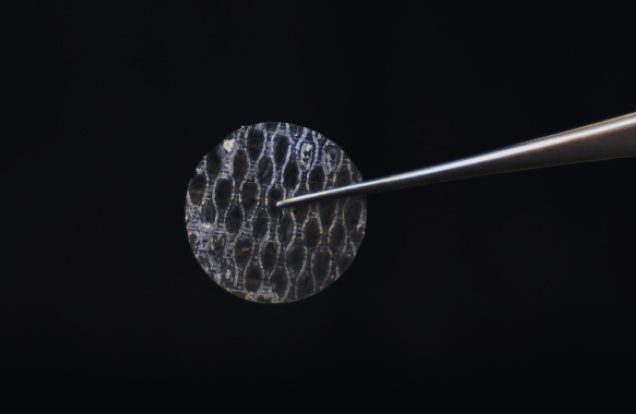
AmnioDisc®
- Lyophilised disc
- Myringoplasty, epistaxis
AMNIODISC®
Current product status: Product under development
Method of administration: topical application, administered by a doctor
Form of product: Sterile deep-frozen product, various sizes
Shelf life: 2 years, room temperature

MECHANISM OF ACTION
EXAMPLES OF USE
Suitable for eye and ear wounds, corneal and ear erosions; neurotrophic ulcerations; acute chemical/thermal burns; non-healing epithelial defects; post-infectious keratitis, Bullous keratopathy, repair of tympanic, membrane perforations.

AMNIOBARRIER®
- Binary gel/ Solid patch
- Unwanted adhesion
AMNIOBARRIER®
Current product status: Product under development
Method of administration: topical application, administered by a doctor
Form: sterile, in gel, different volumes
Shelf life: 2 years, room temperature


MECHANISM OF ACTION
AMNIOBARRIER® is a product in development that will be used primarily as a preventative measure for the development of unwanted adhesions after caesarean section deliveries and small pelvic surgery.
AMNIOBARRIER® is a sterile, single-use, multilayered medical device.
EPISTAR® is an advanced device that acts as a physical barrier to protect internal tissues from unwanted adhesions. This dressing is designed to maintain contact with different tissue types and provide flexible, softly adherent coverage of the surface of various organs. AMNIOBARRIER® acts as a physical barrier to protect organs from unwanted adhesions over the long term, speeding recovery and helping to prevent bacterial contamination.
OUR EXPERIENCE
EXAMPLES OF USE
- Application of cesarean delivery
- Application after surgery in the small pelvis area

AM_Nx
- Cryopreserved patch
- Decompressive craniectomy
AM_NEURO
Current product status: approved and applied from 2015
Form of product: Sterile deep-frozen product without medium
Storage life: 5 years, -40 to -80°C

MECHANISM OF ACTION
AMNIOBARRIER® is a product in development that will be used primarily as a preventative measure for the development of unwanted adhesions after caesarean section deliveries and small pelvic surgery.
AMNIOBARRIER® is a sterile, single-use, multilayered medical device.
EPISTAR® is an advanced device that acts as a physical barrier to protect internal tissues from unwanted adhesions. This dressing is designed to maintain contact with different tissue types and provide flexible, softly adherent coverage of the surface of various organs. AMNIOBARRIER® acts as a physical barrier to protect organs from unwanted adhesions over the long term, speeding recovery and helping to prevent bacterial contamination.
EXAMPLES OF USE
- decompressive craniectomy
- craniotomy
OUR EXPERIENCE
Ongoing clinical study in cooperation with University Hospital Brno. The aim of the clinical study is to verify the uncomplicated and simple intraoperative handling of AMNIO for neurosurgery. Confirmation of the proper functionality and safety of the product and preclusion of postoperative complications resulting from its use.
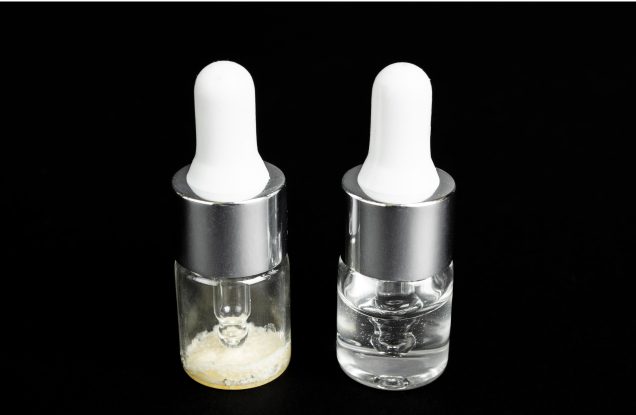
AMx1102
- Lyophilised drops for resuspension
- Dry eye syndrome, conjunctivitis
AMNIODROP
Current product status: Product under development
Application method: topical application
Form of product: Sterile lyophilised product intended for resuspension
Shelf-life: 2 years, room temperature

MECHANISM OF ACTION
AMNIODROP, which works on a base of amniotic fluid, is entirely biological in origin. It contains a large quantity of proteins, growth factors, cytokines, and other specific molecules that are present in perinatal tissue. We make AMNIODROP using the patented AMNIPUR® process, which preserves important biologically active ingredients, while at the same time ensuring maximum safety for the patient. AMNIODROP promotes healing, is non-immunogenic, and uses the natural regenerative and anti-inflammatory effects of amniotic fluid.
EXAMPLES OF USE
- Accelerated regeneration after eye surgery
MARKETING AUTHORISATION 2012
AMNIOEYE®
- Lyophilised transplant
- in ophthalmology in the treatment of More...
AMNIOEYE®
Current product status: Product under development
Application method: topical application
Form of product: Sterile lyophilised product intended for resuspension
Shelf-life: 2 years, room temperature

MECHANISM OF ACTION
EXAMPLES OF USE
- Keratitis of various origin
- Corneal erosion and ulcers
- Bullous keratopathy
- Mechanical and chemical injury to the eye
- Lysis or perforation of the cornea
- In the treatment of fornix adhesions
OUR EXPERIENCE
This product has been widely available on the market for more than ten years now. During this time, we have helped save the eyesight of thousands of patients in many countries.
PUBLICATIONS
- Regeneration and repair of peripheral nerves with different biomaterials M Siemionow, M Bozkurt, F Zor Microsurgery 30 (7), 574-588 2010
- A prospective analysis of free flap monitoring techniques: physical examination, external Doppler, implantable Doppler, and tissue oximetry RF Lohman, CJ Langevin, M Bozkurt, N Kundu, R Djohan Journal of reconstructive microsurgery 29 (01), 051-056 2013
- Current concepts in the management of Marjolin's ulcers: outcomes from a standardized treatment protocol in 16 cases M Bozkurt, E Kapi, SV Kuvat, S Ozekinci Journal of burn care & research 31 (5), 776-780 2010
- A new composite midface allotransplantation model with sensory and motor reinnervation F Zor, M Bozkurt, D Nair, M Siemionow Transplant International 23 (6), 649-656 2010
- Dentition, bone loss, and the aging of the mandible CN Ozturk, C Ozturk, M Bozkurt, HS Uygur, FA Papay, JE Zins Aesthetic surgery journal 33 (7), 967-974 2013
- New composite tissue allograft model of vascularized bone marrow transplant: the iliac osteomyocutaneous flap S Nasir, A Klimczak, E Sonmez, M Bozkurt, S Gibson, M Siemionow Transplant International 23 (1), 90-100 2010
- Variables affecting postoperative tissue perfusion monitoring in free flap breast reconstruction CN Ozturk, C Ozturk, W Ledinh, M Bozkurt, G Schwarz, C O'Rourke, ... Microsurgery 35 (2), 123-128 2015
- Evaluation of the effect of thymoquinone treatment on wound healing in a rat burn model CT Selçuk, M Durgun, R Tekin, lyas Yolbas, M Bozkurt, C Akçay, ... Journal of Burn Care & Research 34 (5), e274-e281 2013
- Composite osseomusculocutaneous sternum, ribs, thymus, pectoralis muscles, and skin allotransplantation model of bone marrow transplantation M Bozkurt, A Klimczak, S Nasir, F Zor, L Krokowi̇cz, M Siemionow Microsurgery 33 (1), 43-50 2013
- The reconstruction of full-thickness ear defects including the helix using the superior pedicle postauricular chondrocutaneous flap CT Selçuk, M Durgun, M Bozkurt, V Kinis, M Özbay, S Bakir Annals of Plastic Surgery 72 (2), 159-163 2014
- Increasing the survival of transverse rectus abdominis musculocutaneous flaps with a Botulinum toxin-A injection: A comparison of surgical and chemical flap delay methods G Temiz, N Yeşiloğlu, H Şirinoğlu, AC Akpınar, M Sarıcı, D Filinte, ... Journal of Plastic, Reconstructive & Aesthetic Surgery 69 (7), 944-951 2016
Licence Partners


Contacts
KHERION
Harbiye, Mim Kemal Oke St. No:29 Flat:2 Maçka/Nişantaşı/İstanbul
+90 533 665 7789
info@kherion.com.tr

CONTACT FORM